Inside EU Health: Trilogue horse trading to the EU's global talent search
Pharma reforms, tobacco taxes, patient concerns, and Europe's aging population.

Brussels is gearing up for more talks on the Pharma Package, with negotiations over EMA governance and environmental rules for medicines tied to market authorisation timelines. Industry voices warn that half-measures risk undermining innovation, while major European companies push for lighter regulation in exchange for fresh investment pledges.
Pharma Package: Yesterday’s Coreper meeting centered around preparations for next week’s second trilogue (Commission, Council, European Parliament) on 7 October, which will look at the questions of European Medicines Agency governance.
The Danish Presidency put forward some potential trade offs that could bring the Parliament and Council closer together in their views.
The Parliament wants to extend the scope of the Environmental Risk Assessment to include all pharmaceuticals, whereas the Council wants to restrict it to the manufacturing of antimicrobials. The trade off is linked to the seemingly unrelated deadlines for market authorisation, where the Council wants to revert to 210 days and the Parliament wants to limit it to 180 days. The document says that while the two are not interlinked, they are related in terms of workload of administrations.
Half measures v. full ambition: In a guest blog, Sini Eskola of Takeda and Nick Sykes, a Policy Advisor for Regulatory Strategy at EFPIA, Brussels’ largest pharma lobby, argue that if done right, the GPL (Pharma Package - General Pharmaceutical Legislation) can protect patients, accelerate access to new treatments, and secure Europe’s leadership in global health innovation.
They argue that evidence shows some proposals - such as faster assessments and regulatory sandboxes - strengthen agility and support innovation, while others risk limiting effectiveness if not refined, particularly around support for combination products (drug-device/diagnostic/companion diagnostic), a lack of flexibility in utilizing different types of evidence such as ‘real-world evidence’, and the exclusion of marketing authorisation holders from labelling decisions.
Two other issues are highlighted. Implementation and alignment with the Biotech Act and Innovation Act. Read the blog here.
Copenhagen pledge: Meeting in Copenhagen with Ursula von der Leyen, Emmanuel Macron, Donald Tusk, and Mette Frederiksen, 28 of Europe’s largest companies, including drug-maker Novo Nordisk, have pledged to increase investments in Europe by 50% by 2030. Their condition? The investment depends on continued reforms, namely: simpler rules, faster approvals, and greater support for green energy and innovation. All quite easy to say, but more difficult to deliver.
Novo Nordisk is a leading member of EFPIA, which has been highly critical of the Pharma Package.
CEO of the Confederation of Danish Industry, Lars Sandahl Sørensen,said that heavy regulation is “slowing down European companies and preventing them from competing with other global giants.”
Taxing ‘toy-like’ vapes: Tax Commissioner Wopke Hoekstra’s LinkedIn post criticizes the “covert ways” in which the tobacco lobby operates and said that they try to downplay the risks of tobacco replacement products, in “the exact same way they downplayed the risks of light cigarettes in the past”. The post comes ahead of discussions with the ECOFIN Council (10 October) on his updated Tobacco Tax Directive. Read more here.
Wanted! Patients’ perspectives: The European Medicines Agency (EMA) has released a draft reflection paper on patient experience. EMA points to the value of patient experience, which isn’t always truly reflected in clinical trials. They offer the example of cancer where patients may want to prioritise quality of life over traditional clinical endpoints like overall survival. The goal is to further improve evidence generation and optimize future medicine development towards outcomes that matter most to patients. The consultation is open until 31 January 2026.
Safe and accessible abortion: The My Voice, My Choice campaign has delivered 1.1 million signatures to the European Commission, calling for safe and accessible abortion across the EU. Campaigners from countries including Malta and Italy shared how legal bans and systemic barriers continue to deny women care, while urging Brussels to act on their European Citizens’ Initiative. The Commission is expected to respond by March 2026. Read more here.
Choose Europe: The European Commission has launched the first call for Choose Europe for Science – grants with €22.5 million in funding, it is part of a €500 million envelope for 2025–2027 announced in May. The initiative was in part motivated by the slashing of research grants in the US and the possibility of Europe benefitting from attracting what President von der Leyen calls, “the best and brightest”. Find the link here.
PCI Pharma: The European Commission has approved the acquisition of joint control of KPCI Holdings Limited, operating as PCI Pharma Group, which is an end-to-end provider of services to pharmaceutical and biotech companies (CDMO), by Bain Capital Investors, LLC and Kohlberg & Co., L.L.C., both based in the United States.
What we’re reading
‘Your next medical visit could be with a banned doctor’: Follow The Money’s Joline de Vries investigates how doctors banned for serious medical malpractice in one EU country can move to another state and continue to work. FTM’s investigation identified dozens of cases. Read their article here.
Germany’s health-driven debt: Der Spiegel reports on the 2025 Over-Indebtedness Report by the Hamburg Institute for Financial Services (IFF), which finds that health problems are the leading cause of long-term financial distress in Germany, with one in six individuals citing illness as the main reason for their over-indebtedness. The report highlights that singles and single parents are especially vulnerable, as the financial strain of medical issues often leaves them unable to meet ongoing obligations.
Chart of the day
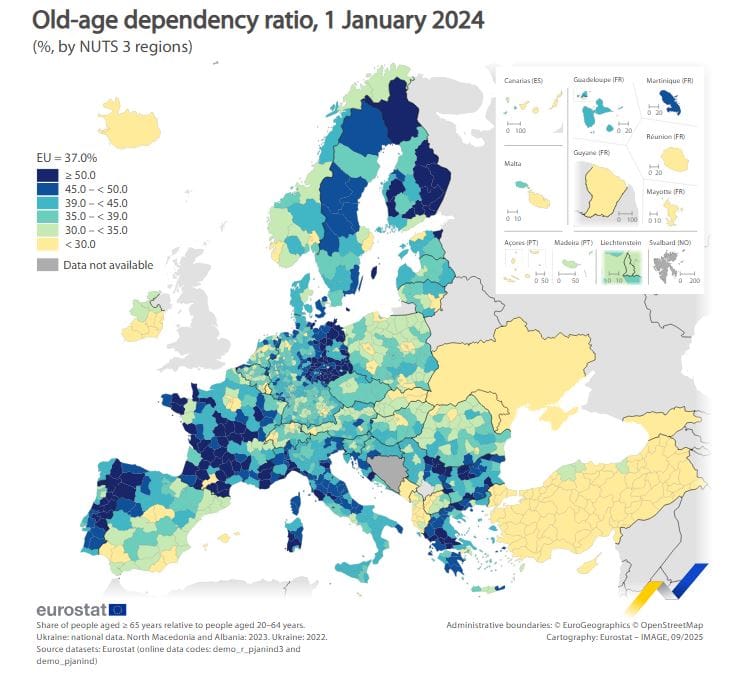
The EU’s old-age dependency ratio - measuring the share of people aged 65 and over compared with the working-age population (those aged 20 to 64) - has risen significantly over the past two decades. In 2004, the ratio stood at 26.8%, meaning there were just under four working-age adults for every person aged 65 or above. By 1 January 2024, it had climbed to 37.0%, leaving fewer than three working-age adults per elderly person. This has many knock on consequences for pension sustainability and healthcare.
