Inside EU Health: CMA trilogue; cardiovascular strategy; EMA medicines approval; healthcare accessibility
Critical Medicines Act negotiations begin; prevention takes centre stage in opening EU cardiovascular health report; EMA recommends approval of six medicines, expands indications for nine; Eurostat figures show wide gaps in healthcare accessibility across EU

Critical Medicines Act negotiations begin, with urgency stressed by co-legislators
The kick-off trilogue on the Critical Medicines Act (CMA) took place on Tuesday evening, with talks described as constructive and urgent, as co-legislators signaled readiness to move swiftly.
Earlier, Cypriot Health Minister Neophytos Charalambides told MEPs the file was a presidency priority, saying a deal could be reached within six months amid geopolitical pressures.
The first trilogue focused on exchanging mandates, with key issues including scope, strategic projects, stockpiling, joint procurement and financing. Technical talks will now prepare the next trilogue on 16 March.

First exchanges set the tone for EU cardiovascular health debate
The European Parliament’s first debate on Romana Jerković’s draft report on an EU cardiovascular disease strategy showed broad consensus on the need for action. Jerković stressed the urgency of tackling the EU’s leading cause of death through prevention, stronger primary care and addressing inequalities, including harmful marketing to children.

MEPs across groups echoed support for prevention, while highlighting priorities such as youth-focused measures, innovation, treatment, environmental risks and multimorbidity.
The Parliament’s position aims to toughen the Commission’s proposal, particularly on prevention.

EMA recommends approval of six medicines, expands indications for nine
The European Medicines Agency’s (European Medicines Agency) human medicines committee (CHMP) has recommended six medicines for approval and expanded the use of nine others at its January meeting.
Among the highlights, the committee backed Fylrevy, a new hormone replacement therapy for postmenopausal women without a uterus. Hungarian maker Gedeon Richter, said it is the first innovative menopause treatment approved in Europe in decades.
The CHMP also approved the first-ever treatment for ultra-rare thymidine kinase 2 deficiency, supported through EMA’s PRIME scheme, marking a major breakthrough for patients.
Not all decisions were positive. EMA declined to grant a new heart-failure indication for Lilly’s GLP-1 drug Mounjaro, though supporting data will be added to product information. By contrast, Novo Nordisk’s semaglutide received conditional approval for treating fatty liver disease (MASH). Finally, regulators launched a data-integrity review of the autoimmune drug Tavneos.

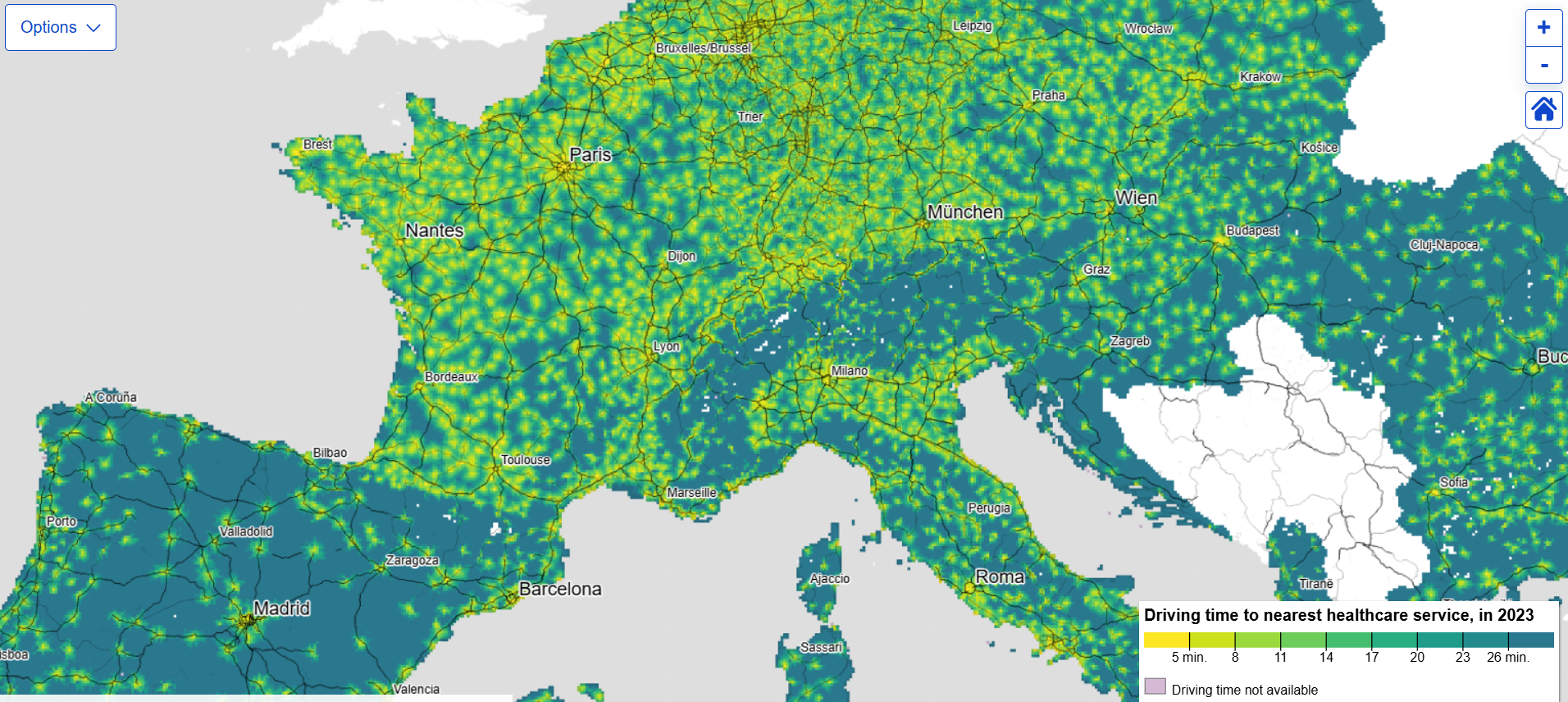
Eurostat figures show wide gaps in healthcare accessibility across EU
Access to healthcare is a key determinant of health and a central concern of the Sustainable Development Goals. Accessibility datasets from Eurostat, published today (2 February) on a 1 km resolution grid, measure road travel time from populated places to services such as healthcare facilities, highlighting how location and transport affect access to care.
These indicators support monitoring of SDG 3 on good health and well-being, while also informing SDG 10 on reduced inequalities and SDG 11 on sustainable cities and communities by revealing spatial disparities. As the data rely on heterogeneous national registers, some inconsistencies may exist, and Eurostat invites users to provide feedback to help improve data quality and relevance.