Inside EU Health: Climate inaction and health; ACT EU goes multilingual; and the US sets new rules for biosimilars
Climate inaction and health; ACT EU goes multilingual; and the US sets news rules for biosimilars
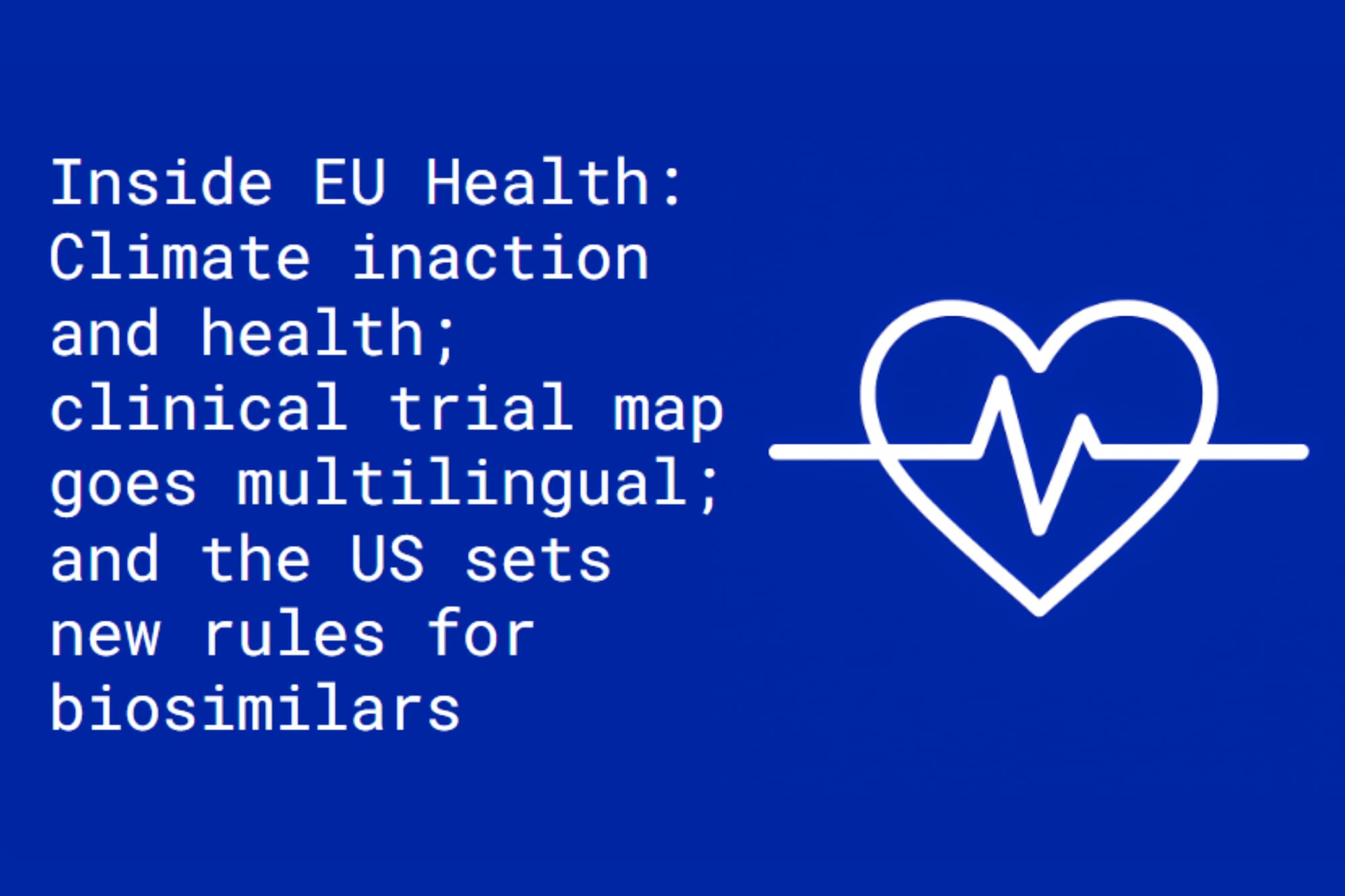
Human cost of climate change: The 2025 Lancet Countdown report warns that escalating climate inaction is driving a global health crisis. Heat-related deaths have surged 23% since the 1990s, with over half a million people dying each year from extreme heat.
Despite mounting evidence of the health toll, fossil fuel expansion and deforestation continue apace. The world’s 40 largest private banks poured a five-year high of $611 billion into fossil fuel projects in 2024—up 29% from the year before.
“If we remain locked into fossil fuel dependence, health systems, cooling infrastructure, and disaster response capacities will soon be overwhelmed,” Professor Nadia Ameli, Co-Chair of the Lancet Countdown Working Group, said.
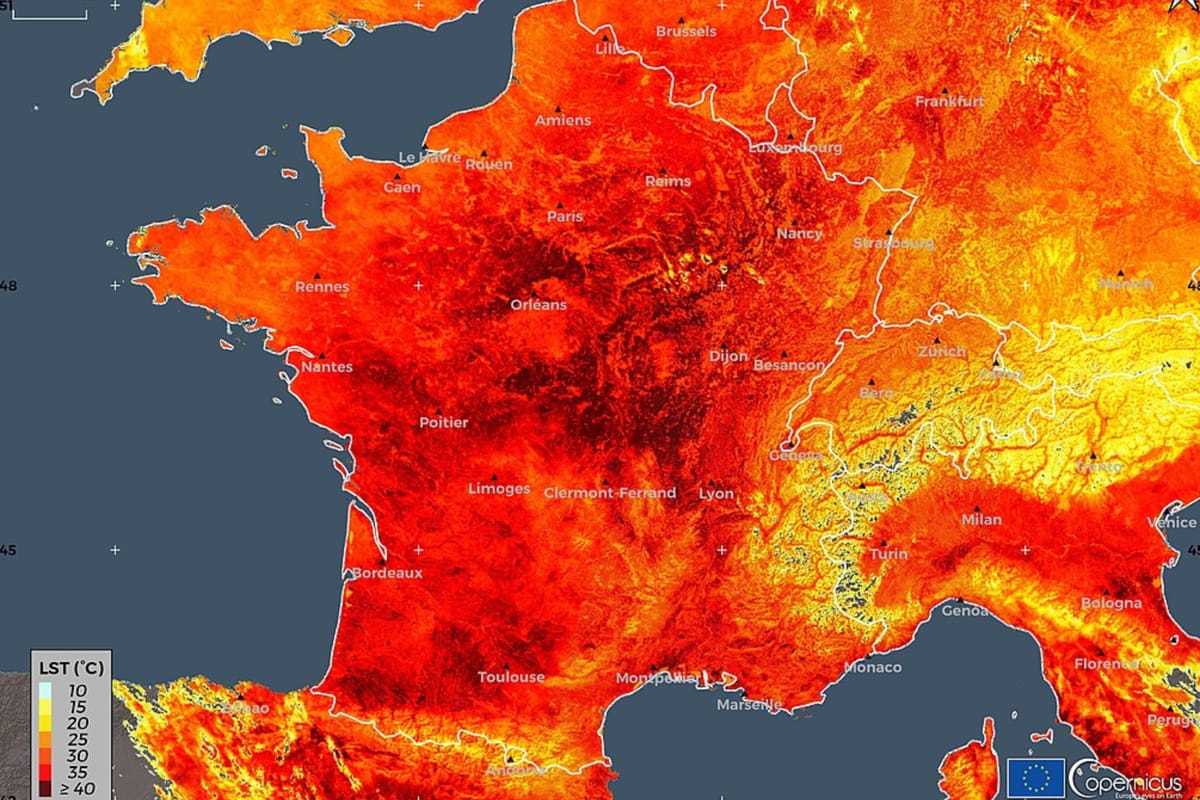
ACT EU goes multilingual: The Accelerating Clinical Trials in the EU (ACT EU) interactive map is now available in all EU languages. You can select your language, medical condition and country to show where trials are taking place, and can select which are recruiting. Here’s a search in Irish.
The interactive map is open to everyone to search and offers patients, healthcare professionals and researchers a quick up-to-date overview of clinical trials across the EU as well as Norway and Iceland. By clicking on a dot you can find out more about the nature of the trial and contact details.
The information on the map comes from the Clinical Trials Information System (CTIS), where all clinical trials in the EU must be registered since January 2023. Since the CTIS became mandatory, an average of 200 new clinicals trials were submitted every month. Of these, around 80 applications per month are for multinational clinical trials.
Biosimilar blast off? The US Food and Drug Administration (FDA), which is equivalent to the European Medicines Agency (EMA), has announced that it is making biosimilar approval faster and less expensive.
FDA Commissioner Marty Makary says the measures could significantly lower health care costs in the US, saying, “We can achieve massive cost reductions for advanced treatments for cancer, autoimmune diseases, and rare disorders affecting millions of Americans.”
The FDA is proposing to reduce, as it describes, unnecessary clinical testing and studies that biosimilars are currently subject to. Biosimilars are not identical to the biologic drug they replace, but they must demonstrate that they are as safe and effective as the original product.
The US HHS (Health and Human Services) says that biologic medicines account for 5% of US prescriptions but 51% of total drug spending, according to figures for 2024. Apart from faster approval, the FDA also proposes to make it easier for pharmacists to choose a biosimilar over its branded equivalent.
US lags behind the EU: The FDA has approved 76 biosimilars to date, while EMA has approved more than 140 since 2006. In 2022, EMA and the Heads of Medicines Agencies (HMA) issued a joint statement stating that biosimilars should be considered interchangeable with branded biological medicines; up until then, not all EU states took this view.

