Inside EU Health: Biotech Act(s); WHO preparedness; and study on drug spending dynamics
Biotech Act(s); WHO preparedness; EMA consults on primates in research; and IQVIA study on drug spending dynamics

Biotech, a play in two acts: The EU’s Biotech Act will land in two stages, with a health-focused proposal this year and a broader framework in 2026.
Von der Leyen pledged “new European legislation on biotechnology in 2025,” and Commissioner Olivér Várhelyi has been determined to deliver. He has repeatedly insisted the Biotech Act would be published this year. His confidence now appears justified - at least in part.
The first Biotech Act will focus on health innovation and simplifying clinical trial and medical device rules. A second, broader initiative will follow in 2026.

WHO preparedness guidelines: The World Health Organization (WHO) has released a comprehensive guide to help countries strengthen their preparedness and response to health emergencies. The ‘National Health Emergency Alert and Response Framework’ has been developed with input from global experts and is informed by real-world experiences, including the 300 recommendations that were made following the COVID-19 crisis.
The guide builds on the ‘7-1-7’ evaluation framework, which sets performance targets of 7 days to detect an outbreak, 1 day to notify public health authorities, and 7 days to complete early response actions.
Consultation on use of primates in testing: The European Medicines Agency has published a reflection paper on the use of non-human primates (NHPs) in the safety testing of medicines and diagnostics.
European legislation only allows non-human primates (NHP) to be used in research related to debilitating or potentially life-threatening clinical conditions in humans and where there is a scientific justification that the research cannot be achieved through the use of other species.
The paper makes several recommendations and concludes that while complete replacement of NHP may remain challenging, accumulating evidence indicates that replacement is often achievable.
The consultation is open until 31 January 2026.
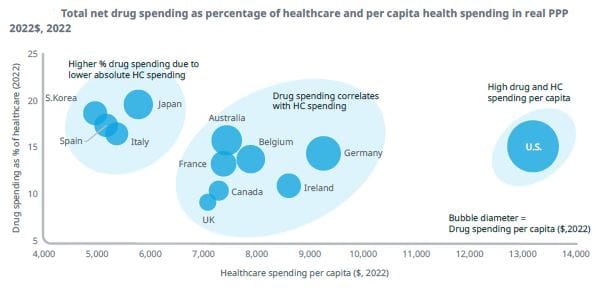
Medicine spending is stable at around 15%: The IQVIA Institute - the research arm of the well known medicine research service company, has published a report looking at drug expenditure over the 2000 - 2022 period across 12 major markets.
As health care systems and payment systems vary widely, the report uses total drug expenditure to compare 12 major markets. The ‘total drug expenditure’, includes prescription and over-the-counter medicines, from all channels and uses prices reported after discounts and rebates.
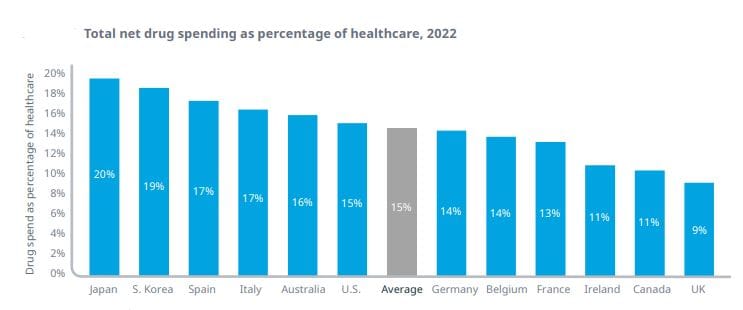
The authors acknowledge that there are significant variations in everything from health needs to economic resources, but observe some trends. For example, drug expenditure tends to be higher as a percentage of overall health spending when a country spends less on healthcare.