EU-India free trade deal will slash tariffs for medicines and medical devices
The EU and India have agreed a free trade deal that promises to significantly cut tariffs on medicines and medical devices

The European Union and India have concluded negotiations on a landmark Free Trade Agreement (FTA), with Brussels signalling that the outcome surpasses several of the EU’s recent trade deals. A senior European Commission official said the agreement is “considerably better” than those concluded with Australia, EFTA and the UK.
According to the official, the turning point came when the full College of Commissioners travelled to India in February of last year and publicly committed to finalising the deal by the year’s end. That deadline ultimately slipped, largely because the EU prioritized completing its long-running negotiations with Mercosur countries (Argentina, Brazil, Paraguay, and Uruguay).
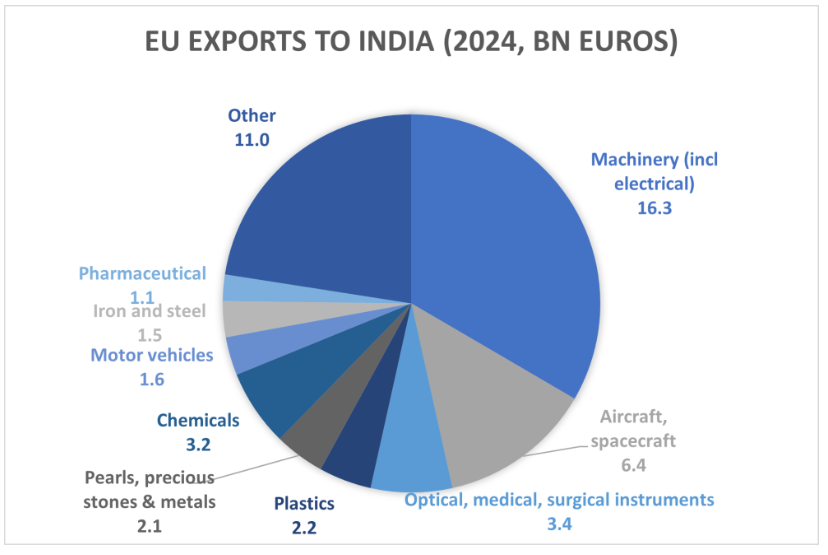
Both sides are presenting the agreement as a win for the pharmaceutical and medical device industries, among other sectors. India has agreed to largely eliminate tariffs of up to 11% on pharmaceuticals and on 90% of “optical, medical and surgical equipment”, where duties can reach as high as 27%.
In 2024, EU exports to India amounted to just €1.1 billion in pharmaceuticals and €3.4 billion in optical, medical and surgical equipment. By contrast, India’s pharmaceutical exports to global markets were worth an estimated €24.9 billion in 2025. Whereas India is the leading producer of generics, the EU specializes in higher-value products.
Intellectual property rights, a long-standing stumbling block in EU–India trade talks, appear to have been handled with greater pragmatism this time. Both parties reaffirmed their commitment to the TRIPS Agreement and the Doha Declaration, which places access to medicines ahead of purely commercial considerations.
The Commission official said the agreement includes a “strong” intellectual property chapter, offering protection “a little bit above TRIPS in the WTO”, while still allowing for enforcement. This marks a shift from earlier EU negotiating positions that pushed for “TRIPS-plus” provisions, including extended data exclusivity periods favoured by European pharmaceutical companies.
Pavan Choudary, chair of the Medical Technology Association of India (MTaI), said the deal reflects complementary strengths on both sides. “India’s pharmaceutical sector anchors the global supply of generic medicines, while Europe leads in advanced therapeutics, clinical research and regulatory science,” he said. “If carefully aligned, these strengths can support co-development ecosystems that improve affordability, accelerate innovation and strengthen supply security for both partners.”
Asked if they had any concerns about the deal, Medicines for Europe, the association for generics, biosimilars and value-added medicines, told Vital Signs that they were looking forward to seeing the full details of the EU-India FTA before taking a formal position. However, they said they understand that progress has been made in eliminating tariffs on most medicines, which they welcome as a positive development.
Likewise, EFPIA (European Federation of Pharmaceutical Industries and Associations) is awaiting the final text before commenting. However, EFPIA's criticism of the Mercosur trade deal's lack of "meaningful access" to public procurement, will likely apply to the EU-India deal, which does not have a chapter on procurement. There is also likely to be concern about protecting intellectual property.
Next, the European Union will move from negotiation to formal approval by publishing the negotiated draft texts, followed by legal scrubbing and translation into all official EU languages. The European Commission will then propose the EU-India FTA to the Council. The agreement will be formally signed by the EU and India, and subsequently submitted to the European Parliament for its consent. Assuming Parliament approves, the Council will make the final decision to conclude the agreement, allowing it to enter into force. A note to the wise, the European Parliament's approval can never be taken for granted.
