EMA says ‘no new evidence’ to support changes to HRT warnings
The European Medicines Agency (EMA) says it has seen no new evidence that points to a need to amend the current labelling of HRT medicines

On Monday (10 November), the US Department of Health and Human Services (HHS), led by Robert Kennedy, announced that the US medicines regulator, the FDA, would initiate the removal of ‘black box’ warnings from hormone replacement therapies (HRT). Black box warnings are used to indicate that a medication may present serious risks.
“For more than two decades, bad science and bureaucratic inertia have resulted in women and physicians having an incomplete view of HRT,” said Secretary Kennedy. “We are returning to evidence-based medicine and giving women control over their health again.”
The FDA is now working with companies to update language in product labelling to remove references to risks of cardiovascular disease, breast cancer, and probable dementia. However, it will maintain the boxed warning for endometrial cancer for oestrogen-only products.
‘Gold-standard science’
Kennedy has a long history of ignoring evidence, most notably for undermining confidence in childhood vaccination and linking the MMR vaccine to autism. This claim has been repeatedly debunked and is based on fraudulent research by Andrew Wakefield.
More recently, Kennedy linked the use of Tylenol (paracetamol) to autism, leading the EMA to issue a statement reassuring women that it had found no evidence that taking paracetamol during pregnancy causes autism in children.
Kennedy and the FDA Commissioner Marty Makary argue that women have been denied the benefits of hormone replacement therapy because of “medical dogma rooted in a distortion of risk.” They cite studies from 1991, 1996 and 1980, which they say show that HRT may reduce the risk of cardiovascular disease, Alzheimer’s and bone fractures, respectively.
EMA says that in the EU, product information for hormone replacement therapy (HRT) medicines remains unchanged: "There is no new evidence to suggest that the labelling of HRT products in Europe needs to be amended."
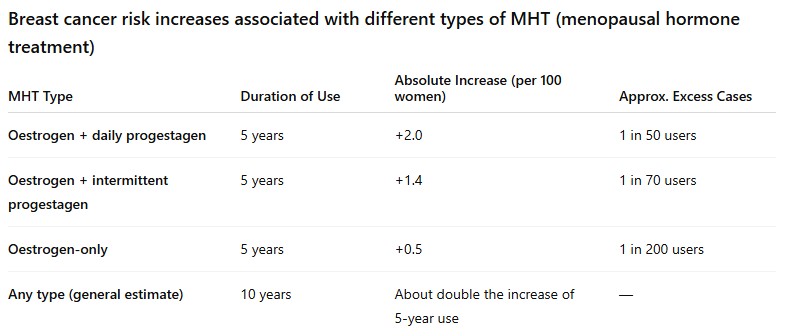
The EU has a different labelling system, where a ‘warnings’ and ‘contraindications’ section is included in a medicine’s product information. The current product information includes the risk of breast cancer and links it to the duration of exposure.
In 2020, EMA’s safety committee recommended updating the safety information for HRT. The updates were based on evidence from a large study published in The Lancet in 2019. The study confirmed the known higher risk of breast cancer in women using HRT. The results also showed that the risk may continue to be higher for ten years or more after stopping HRT, if HRT is used for more than five years.
Since the last update in 2020, EMA has continuously monitored the safety of HRT, including through periodic safety update reports (PSURs). EMA said that these reports ensure that all the safety information available on authoriced medicines is regularly reviewed, even if no concerns have been raised from any source.
The EU product information includes information on the risk of other conditions, including ovarian cancer, venous thromboembolism, ischaemic stroke and probable dementia. It also mentions the increased risk of endometrial cancer associated with oestrogen-only HRT.
Ultimately, these decisions are made by a doctor and tailored to each woman’s individual situation, usually taking into account of family history, weight, age at first birth, and other factors, such as kidney and liver conditions - among others.
EMA says that national competent authorities in the EU continue to monitor the safety of HRT and promptly evaluate any new data as it emerges, "regulatory actions are taken when needed to protect public health."
